Diabetes Workshop Report
100 Years of Insulin: What’s Next?
Table of Contents
- Message from CIHR Institute of Nutrition, Metabolism and Diabetes Scientific Director, Norman Rosenblum
- Workshop Objectives
- The Disruptors
- Partner Presentations
- Small Group Discussions
- Conclusion and Closing Remarks
- Appendix 1: Agenda
- Appendix 2: Meeting Participants
- Appendix 3: CIHR Scientific Directors And Staff
Message from CIHR Institute of Nutrition, Metabolism and Diabetes Scientific Director, Norman Rosenblum
Dr. Fredrick Banting and Dr. John Macleod were awarded the Nobel Prize in Physiology or Medicine in 1923 for the discovery of insulin and its use in the treatment of type 1 diabetes. Today, I am proud to provide leadership for the CIHR Institute of Nutrition, Metabolism and Diabetes (CIHR-INMD), which provides a focus for Canadian diabetes investigators who are internationally recognized for their excellent work. The 100-year anniversary of this ground-breaking discovery will be marked in 2021; this represents an important moment to reflect on how our understanding of diabetes has improved over time and to consider how we might invest strategically in new diabetes research to improve the quality of life and health outcomes for Canadians living with diabetes.
Over the past century, our understanding of diabetes has increased dramatically, as have the options for treatment. However, more work needs to be done. The global prevalence of diabetes is 8.8%, and this rate is expected to increase world-wide largely as a result of the aging population and increasing obesity rates. While these factors are contributing substantially to the dramatic increases in type 2 diabetes, there is also evidence of increasing prevalence of type 1 diabetes.
The 100 Years of Insulin: What's Next? Workshop was held in conjunction with the 2018 Diabetes Canada (DC)/ Canadian Society of Endocrinology and Metabolism (CSEM) Professional Conference, October 10, 2018 in Halifax. The Workshop brought together invited Canadian diabetes investigators who work in areas related to mechanistic and therapeutic research with internationally recognized research leaders from other countries. We asked these highly-esteemed "disruptors" to challenge our ideas about how we can advance diabetes research and be truly innovative in our strategic focus. I wish to thank these individuals for engaging with us and for generously participating in the Workshop.
Thank you to my CIHR Scientific Director colleagues, Drs. Charu Kaushic from the Institute of Infection and Immunity and Chris McMaster from the Institute of Genetics, as well as the other Institute representatives who participated in this Workshop. I also wish to thank partner organizations for their participation and valuable insights. In particular, I wish to thank Diabetes Canada for providing us an opportunity to host this Workshop in conjunction with their annual scientific meeting. We look forward to continuing the discussions that began at this Workshop to develop partnerships, leverage CIHR investments and contribute to mobilizing capacity across the Canadian health research enterprise to impact the health of Canadians so that in another 100 years, we will have another significant milestone to celebrate!
Norman Rosenblum, MD, FRCPC
Scientific Director, CIHR-INMD
Workshop Objectives
The purpose of the workshop was to seek input from the Canadian diabetes research community on the foci, structure and partnership opportunities to inform a strategic research initiative to commemorate the 100th anniversary of the discovery of insulin.
Objectives
The primary objectives of this Workshop were to bring together researchers and other stakeholders to:
- Identify the Canadian strengths from within and external to the diabetes research community that could be leveraged to support a strategic research initiative on the mechanistic and therapeutic aspects of diabetes research.
- Define the scientific priorities of the Canadian diabetes strategic research initiative.
- Identify the potential structures needed to enhance collaboration and advance research in the field.
- Identify a list of potential partners, including international collaborators, who could work with CIHR to support this research initiative.
The Disruptors
Daniel Drucker
Professor of Medicine, University of Toronto

Dr. Drucker received his M.D. from the University of Toronto in 1980, and is currently Professor of Medicine, the Banting and Best Diabetes Centre-Novo Nordisk Chair in Incretin Biology at the University of Toronto and a Senior Scientist at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital. His laboratory studies the molecular biology and physiology of gut hormones with a focus on the glucagon-like peptides. Dr. Drucker's scientific studies identified multiple novel mechanisms of hormone action, enabling development of new drug classes for diabetes, obesity and intestinal failure. His discoveries have been recognized by numerous learned societies.
Dr. Drucker began by reflecting on how far we have come in the past five years in terms of therapies for treating diabetes. He shared data on the effects of empagliflozin, an inhibitor of sodium-glucose cotransporter 2 (an SGLT2 Inhibitor), demonstrating patients with type 2 diabetes at high risk for cardiovascular events who received empagliflozin, as compared with placebo, had a lower rate of a cardiovascular adverse outcome and death. In addition, newer glucagon-like peptide-1 receptor (GLP-1R) agonists, exemplified by semaglutide, also reduce the rate of major cardiovascular events, and result in 1.5% reduction in glycated hemoglobin (HbA1C) and a 3-6 kg weight loss.
Figure 1: EMPA-REG Outcome: Primary and Secondary Outcomes
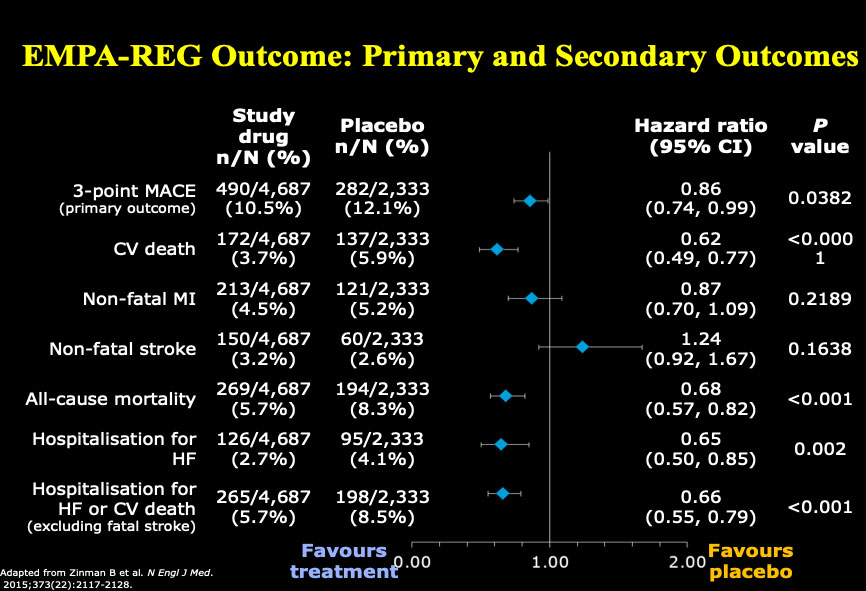
Adapted from Zinman B et al. New Engl J Med. 2015;373 (22): 2117-2128.
Reproduced with permission and presented at this diabetes workshop by Dan Drucker.
Figure 1 – long description
| Study drug n/ N (%) |
Placebo n/N (%) |
Hazard ratio (95% CI) |
P value |
|
|---|---|---|---|---|
| 3-point MACE (primary outcome) |
490/4,687 (10.5%) |
282/2,333 (12.1%) |
0.86 (0.74,0.99) |
0.0382 |
| CV death | 172/4,687 (3.7%) |
137/2,333 (5.9%) |
0.62 (0.49, 0.77) |
<0.0001 |
| Non-fatal MI | 213/4,687 (4.5%) |
121/2,333 (5.2%) |
0.87 (0.70,10.9) |
0.2189 |
| Non-fatal stroke | 150/4,687 (3.2%) |
60/2,333 (2.6%) |
1.24 (0.92, 1.67) |
0.1638 |
| All-cause mortality | 269/4,687 (5.7%) |
194/2,333 (8.3%) |
0.68 (0.57, 0.82) |
<0.001 |
| Hospitalization for HF | 126/4,687 (2.7%) |
95/2,333 (4.1%) |
0.65 (0.50, 0.85) |
0.002 |
| Hospitalization for HF or CV death (excluding fatal stoke) |
265/4,687 (5.7%) |
198/2,333 (8.5%) |
0.66 (0.55, 0.79) |
<0.001 |
However, Dr. Drucker noted there are still unmet needs in type 2 diabetes (T2D), for example:
- Weight loss story is key – will revert and remit disease
- There has also been little progress in complications beyond heart and kidney disease and we don't understand molecular mechanisms
- Insulin resistance, which goes hand-in-hand with obesity, but even though there are weight loss drugs available there is more to do
- Remission (vs. cure; need to keep taking drugs forever)
- Prevention
- A huge challenge is model organisms – the mouse trap: how one rodent rules the lab – investigators have been terribly misled by modeling disease in mice (could be a subject for an entire workshop) – need to be much more critical – we often overstate the utility of model organisms for studies in human metabolic disease
Furthermore, there are unanswered questions about whether the brain is a therapeutic target for diabetes. We should not pretend all central nervous system (CNS) manipulations in mice will carry over to humans. Dr. Drucker was also particularly skeptical about spectacular reductionist stories and has written about issues of reproducibility and the need to be critical of experimental models and reagents.
Dr. Drucker also noted the disconnect between innovation, drug approval and implementation, since there are many gaps in implementing new therapies, where the efficacy has been demonstrated but new drugs have not been added to formularies by Governments.
Finally, Dr. Drucker noted huge potential for innovation in type 1 diabetes (T1D). He noted that Canada is extremely strong in regenerative medicine and cell replacement therapy. He believes that engineers are transforming the field, and that the microbiome offers intriguing clues.
Anna Gloyn
Professor, Molecular Genetics & Metabolism, University of Oxford

Anna Gloyn is Professor of Molecular Genetics & Metabolism and a Wellcome Senior Fellow in Basic Biomedical Science based jointly at the Oxford Centre for Diabetes Endocrinology and Metabolism & the Wellcome Centre for Human Genetics at the University of Oxford. She is also the current lead for the Diabetes & Metabolism Theme of the NIHR Oxford Biomedical Research Centre.
The over-arching aim of her research is to identify effective therapeutic targets for type 2 diabetes (T2D) treatment through mechanistic studies of proteins causally implicated in T2D risk through human genetics. To achieve this goal, she works at a genomic level to unlock the effector transcripts at genome wide association study (GWAS) loci using state of the art genomic techniques in recently developed human pancreatic beta-cell models. The goal is to understand what these proteins do, how they contribute to defects in insulin secretion, what networks they are involved in and how we can leverage this new knowledge to identify therapeutic targets and to use existing therapies more effectively.
Leveraging Human Genetic Discoveries
Dr. Gloyn began by commenting on the suitability of pre-clinical models for predicting efficacy and safety of therapeutic targets. She noted that there is natural genetic variation, which perturbs protein function and or expression and this provides a window into causal pathways for disease. Furthermore, she highlighted that by understanding the direction of effect, loss of gain of protein function, it is possible to understand whether protein levels need to be increased or decreased to protect against disease. She discussed how natural genetic variation offers the potential to study the long-term impact of modulating a protein on human health (e.g. are there long-term undesirable on-target effects of protein perturbation which might make targeting the protein unattractive from a safety standpoint). She provided an example of precision medicine that demonstrates proof of concept. As a post-doctoral researcher in Dr. Andrew Hattersleys' lab she discovered a new type of diabetes, Neonatal Diabetes, which results from mutations in a key component (Kir6.2) of the beta-cell ATP-sensitive potassium channel (KATP) channel. This discovery has resulted in a change in the treatment for patients with this variety of Neonatal Diabetes, from insulin injections to oral sulphonlyurea therapy. Furthermore, she also pointed out that common genetic variation in the same gene influences type 2 diabetes (T2D) risk and the very same drugs can be used to treat T2D.
Dr. Gloyn commented on other lines of evidence, noting the example of PCSK9 inhibitors for the treatment of familial hypercholesterolemia. She cited an example, The Future of Humans as Model Organisms – A human phenomic science approach could accelerate personalized medicine (Science Aug. 10 2018: Vol 361, Issue 6402, p. 552-553) as growing recognition that the time is right for human studies with the avalanche of "Big Data" now available. Dr. Gloyn pointed out that the T2D genetic community had now identified over 250 regions of the genome which influence T2D risk and that each of these offers the potential for novel insights into disease biology. She also recommended opportunities offered by the NIH-funded Accelerating Medicines Partnership (AMP), noting that Canada is well represented in this project where the data emerging from human genetic discoveries has been made publicly available and in an accessible format for non-geneticists.
Dr. Gloyn commented on notable Canadian strengths in islet biology, including the MacDonald Human Islet Isolation Programme which distributes high quality human islets and tissue to research groups around the world. Canada also has world leaders in stem cell biology and has contributed to the protocols used by many around the world to generate stem-cell derived beta-like cells. She believes that more work could be done to bring this expertise together with gene editing for disease modelling. Dr. Gloyn highlighted opportunities to exploit existing expertise and resources in Canada to capitalize on the full spectrum of model organisms available for disease modelling which can be deployed depending on the scope and scale of the project. Finally, she recommended funders consider mechanisms for supporting early-career scientists and bringing together national and international scientists from different disciplines, in particular, cell biologists and geneticists, and noted the need to champion more women to ensure they reach their full potential as leaders in the field.
Robert Ratner
Professor, Georgetown University Medical School

Dr. Robert E. Ratner, MD, FACP, FACE is a Professor of Medicine at Georgetown University Medical School in Washington, DC, and recently stepped down after serving 5 years as Chief Scientific & Medical Officer for the American Diabetes Association from 2012-2017. At the Association, he provided leadership and oversight of scientific and medical activities including research, clinical affairs, program recognition and certification, medical information, and professional education. In this capacity, he oversaw the Association’s support of a broad range of professional education activities and the development of the American Diabetes Association Clinical Practice Recommendations, clinical consensus reports, and expert opinions. His research interests include diabetes therapeutics and complications, with an emphasis on translational efforts from controlled trials into community-based practice. He is the author of more than 150 original scientific articles and 20 book chapters.
National Funders, Regulatory Environment and Industry
Dr. Ratner opened his presentation by re-emphasizing the serendipity of basic science that changes the character of clinical research. He highlighted 3 critical areas: national funders, the regulatory environment and industry.
First, he noted that national funders play a key role, because they can put together large studies, such as the US Diabetes Control and Complications Trial (DCCT), a multicenter, randomized, clinical study designed to determine whether an intensive treatment regimen directed at maintaining blood glucose concentrations as close to normal as possible affects the appearance or progression of early vascular complications in patients with insulin-dependent diabetes mellitus (IDDM). The DCCT ended after 10 years in 1993—a year earlier than planned—when the study proved that participants who kept their blood glucose levels close to normal greatly lowered their chances of having eye, kidney, and nerve disease. This trial fundamentally changed diabetes care. Similarly, the Diabetes Prevention Program (DPP), a large multi-centre trial, demonstrated the impact of early intervention in slowing the progression from impaired glucose tolerance to type 2 diabetes.
Dr. Ratner emphasized the importance of thinking of diabetes as a chronic disease, since people are living 30-50 years with diabetes. He noted that a critical piece is TrialNet that takes a variety of approaches to preventing T1D. One area that he feels hasn’t been adequately studied is beta cell preservation in T2D, noting there is a lack of knowledge about beta cell decline. He also recommended that national funders consider obesity research, examining basic mechanisms underlying body weight set-points, and the role of bariatric surgery. Diabetic kidney disease was singled out as a complication for which we have had limited impact, and for which morbidity, mortality and costs are major concerns. He proposed consideration of new trial designs, such as adaptive trials, pragmatic trials, using Bayesian analysis.
He remarked on the challenging regulatory environment. For example, there are no drugs approved for the prevention of diabetes, although metformin shows long-term efficacy. There are no pathways for approval of drugs for prevention. He also noted a major barrier for new drugs since the U.S Food and Drug Administration (FDA) mandated cardiovascular outcome trial (CVOTs) for every new drug. The cost of these trials becomes more expensive as the outcomes become less frequent and higher sample sizes are required to show effects. The FDA revisited this mandate in October 2018, and is expected to release a new guidance sometime in 2019.
Dr. Ratner expressed the view that new insulins represent incremental changes, but that a far more impactful area of pursuit is obesity and brain/hypothalamic functions. He notes that while weight loss can be achieved on a short-term basis, weight regain always follows, and he feels that basic science investigation is needed in this area.
Dr. Ratner concluded his remarks by saying that we need to think of diabetes as a long-term chronic disease, and that while the U.S. has one of the most sophisticated medical systems in the world, it only works for a small proportion of the population.
Matthias Von Herrath
Professor/Director, Type 1 Diabetes, Center La Jolla Institute for Allergy and Immunology

Dr. Matthias von Herrath is committed to clinical translation of immune-based interventions in autoimmune and metabolic diseases, the latter in particular being an exciting emerging field. His expertise and main strength is working at the interface of experimental research to interpret and refine early phase I/II clinical trials in order to optimize strategies for phase 3 trials and drug approval. This comprises translation from various animal models to human interventions, optimization of immunotherapies and their relative ranking, assessment of combination therapies, development of biomarkers as primary or secondary outcomes, induction of antigen specific tolerance in autoimmunity, regulatory cells and clinical T cell assays. In order to be better able to pursue his goal of clinical translation, Dr. von Herrath accepted the position of Vice President and Head of Novo Nordisk’s diabetes Research and Development Center in Seattle in autumn of 2011. At Novo Nordisk, he built the diabetes translational unit, which is based on less conventional and innovative design. In addition, he took on the task of finding new treatments to diabetic kidney disease in 2017.
Understanding disease pathology remains very close to Dr. von Herrath’s heart and Novo Nordisk enabled him to keep an appointment at La Jolla Institute, where he pursues NIH-funded research on the pathology of type 1 and 2 diabetes as part of the national pancreatic organ donor network (nPOD). This is a multinational collaborative effort where data are shared in real time and no intellectual property yet lots of new knowledge on the pathology of type 1 and 2 diabetes is being generated. It is a unique new collaborative paradigm for academic and also industry settings.
Dr. von Herrath began his presentation by speaking on the basic science behind type 1 diabetes. He showed a graph illustrating that beta cell mass doesn’t decrease linearly after diagnosis. Diabetes likely evolves in a remitting-relapsing fashion and noted that there is often no insulitis in antibody positive (AB+) pre-diabetes. He presented another graph illustrating that there is an increase in beta-cell area in the AB+ pre-diabetic stage, however, there is a drop-in proinsulin suggesting a problem in processing. He stated that in order to understand this mechanism, there needs to be more pathology studies in type 1 and type 2 diabetes. He also briefly discussed the intracellular movements of proinsulin in beta cells.
He then explained that there is an upregulation of Major Histocompatibility Complex (MHC) Class 1 in type 1 diabetes; however, its causes are unknown. He noted that the pattern of upregulation in MHC Class 1 is lobular. He presented a figure showing that MHC Class 1 was hyper-expressed in the islets of patients who have had type 1 diabetes for less than 5 years; whereas, for patients with type 2 diabetes, MHC Class 1 was hyper-expressed in the exocrine pancreas. The following are some potential causes of this upregulation in human islets:
- Virus: Enteroviruses are not known to persist very long-term; Herpes viruses could explain lobular progression of diabetes, but not MHC 1
- Neuronal: Herpes virus in dorsal root ganglion? Innervation?
- Cytokines: Maybe interferons
- Vascular: Better vascularization in active islets
- Consequence: Islets would be killed more easily by cytotoxic T-cells (CD8) cells that are found in human islets and recognize antigens in context of MHC I
Dr. von Herrath then spoke about why it has been so difficult to develop an immunotherapy/prevention for type 1 diabetes, and what is being done right now to solve this issue. He noted that there is potential in using plasmid immunotherapy to prevent beta-cell destruction and prevent diabetes. Islet autoimmunity research has to be done to understand the pathogenesis of type 1 diabetes and ongoing TrialNet studies are needed to help fill this gap. He also noted that having an islet model in vivo to test drugs would also be of great benefit and there is a need for more focus on biomarkers. He expressed his view that without collaboration between industry, big pharma and funders, these research gaps will not be filled adequately.

Dr. Norman Rosenblum moderating the discussion between the disruptors
Rebuttals
Disruptors were each given 2 minutes to comment/rebut points made by others
Dan:
- it's entirely possible today to say we don't need a lot of new drugs for T2D – what we need is a better indication of how to use drugs we have, prevent complications and address T1D
- genetics – effect sizes so small – unlikely we will have breakthroughs
- we can now look after 90% of T2D – any new science will be incremental
- the big challenge is how we make a breakthrough in complications and T1D
Rob:
- agrees with glucose lowering drugs
- huge change in hypothalamic set points in altering set points and hunger as opposed to cannabinoid receptors
Anna:
- challenged Dr. Drucker's point related to the effect size: most common variants have modest effects this doesn't mean that larger effects are not possible. A good example here is Kir6.2 (see above) rare large effect variants cause neonatal diabetes whilst common variants of small effect increase risk of type 2 diabetes.
- Although genetic discovery efforts for complications are currently lagging behind those for T1 and T2D she feels strongly that genetics will also deliver biological insights into diabetes complications which may help with identifying novel drug targets and patient stratification.
Matthias:
- agrees with Dr. Drucker – multifactorial situation – sees value in stratifying patients
- looks for new pathways that could have tremendous impact on disease
General Discussion/Comments
In response to a comment that Dan Drucker is too quick to say “mission accomplished in T2D”, when there is still a problem with remission. We need drugs to modify cell fate – isn’t that the hope? Dr. Drucker noted that if you are a pharma company and if there are generic medications that offer 10% weight loss and 2% A1C reduction, further investment is a daunting decision. The low hanging fruit has been harvested – the bar is very high to get a return on investment for new mechanism. He asked which Pharma companies were in the diabetes business 10 years ago? Amgen, Roche, Bristol Myers Squibb, and noted that now they are leaving, partly driven by the cost of CVOTs and a challenging market for pricing and reimbursement. There is a problem developing a new drug unless the outcomes are unbelievably strong.
Judy Fradkin (NIDDK), noted the Restoring Insulin Secretion (RISE) Study in adults will be finished next June (pediatric study is done) – need a better understanding of islet biology in T2D – all diabetes is beta cell dysfunction, so islet biology is so fundamental. There is a basic biology gap in knowledge – how to solve the chicken & egg stress on beta cell. She noted that there is a push to trials, but we have to run smart trials. Genetics could offer a powerful tool of cause and effect and looking at beta-cell dysfunction, we could possibly develop genetic risk scores. Dr. Drucker noted that despite the power of human genetics, advantages from genetics have not powered the recent revolution in T2D – yes for complications.
A question was asked about whether there is a reset in these diseases? There is some precedent in Hepatitis C. A clinician commented that sometimes there are problems that we don’t anticipate (e.g., retrovirals). We always need to strive for a reset or cure. We should never stop trying to intervene, and we shouldn’t underestimate progress made to date. The question was also raised about continuity between federally funded research and industry? Are we limited in our aspirations about the progress made to date?
Other issues raised:
- There is a mechanism in Germany, maintenance excellence clusters that bring clusters of researchers together of different disciplines to break down barriers. This takes a significant commitment. There was a comment that this is a missed opportunity in Canada where large cohorts exist and there are data repositories which offer opportunities to link genetic, gene-environment data, and health services data – linking pillars and incorporating the notion of bench to population and back. Anna Gloyn feels it's an important area; the Oxford National Institute for Health Research (NIHR) Biomedical Research Centre brings together clinicians, basic scientists, healthcare practitioners (HCPs), data analysists and health economists to tackle major health challenges such as diabetes and multi-morbidity.
- Robustness of the science is an important consideration – 65-80% of published papers are not robust – this is a situation where we can do better to save money – everyone needs to address this issue. Funders could have a fair amount of influence, but we need to change incentives. Impact Factors of journals need to be changed and people need to be rewarded for publishing negative results e.g., 10 years of biomarker research with negative results.
- A missing part of research is patient voices – patients are extremely concerned about hypoglycemia, an area where there is not enough research – this is a huge unmet need
- The limitations of animal models were also discussed further in the context of kidney research – the mouse model is used for kidney research because it is easy to use, but we need better ways of doing research. There are no new tools for nephrologists, so only incremental advancements have been made, e.g., how to improve dialysis
- The potential of cell therapy was discussed – Dan Drucker and Matthias Von Herrath expressed enthusiasm, and feel this is a fantastic area of research, and an unmet need – potentially transformative and investors are keen – Dr. von Herrath noted there are challenges since beta cells need a fair amount of oxygen, and the immune system is a challenge
- It was suggested that we think of ways to move the dial – US colleagues have federally funded infrastructure – in Canada we don't have human islet bank or biobank – something to think about, possibly through a "core" (Norm Rosenblum highlighted the Canadian Microbiome Initiative 2 (CMI2) launch of a national research core)
- Gary Lewis - translational research is about using implementation science and engaging the population/patients, health economists, policy-makers – this is something that he hasn't heard today
- A question was raised about "Precision Lifestyle Habits" and behavioural economics and how the same attention could be focused on lifestyle as to drug therapies – Norm Rosenblum responded that this is a Workshop on mechanisms and therapeutics – there are many dimensions to this discussion and this is about making choices about foci of investment
Partner Presentations
Jessica Dunne
Director of Research, JDRF

JDRF is focused on delivering therapies that will lead to better outcomes for all individuals with or at risk of developing type 1 diabetes (T1D) or its complications. JDRF aims to support research and catalyze therapeutic developments that will cure, prevent and better treat T1D. Our focus and funding span the research continuum, i.e., discovery, development and delivery of drugs and devices to people with T1D.
Jessica Dunne, Ph.D., is the director for JDRF’s Prevention program, which aims to delaying or preventing T1D through addressing scientific gaps including the investigation of potential triggers, biomarkers to predict risk and rate of progression and clinical interventions to delay or prevent T1D. Recently, she has initiated work into understanding how machine learning can be applied to biological questions in T1D. She currently serves as the co-director for the T1D Consortium project, which aims for the regulatory qualification of islet autoantibodies.
Dr. Dunne started by explaining the name change from the Juvenile Diabetes Research Foundation to simply “JDRF” to reflect that type 1 diabetes is not a childhood disease. Nowadays, many adults are diagnosed with type 1 diabetes and most people living with the type 1 diabetes are adults.
She then focused her remarks on JDRF’s priorities. JDRF is including patients in the research process, since the end goal of the research is to produce change in the lives of patients. Lastly, she noted the 7 program areas that JDRF is focusing its research on: artificial pancreas, glucose control, complications, beta cell replacement, regeneration therapies, immune therapies and prevention.
Elisabeth Fowler
National Director of Research, Kidney Foundation of Canada

As National Director of Research for the Kidney Foundation of Canada (KFOC), Ms. Fowler is responsible for leading KFOC’s research program, for communicating the outcomes and impacts of kidney research to internal and external stakeholders, and for engaging with patients, providers and researchers in the development of research strategies.
Ms. Fowler opened her presentation by giving a brief overview of the Kidney Foundation of Canada (KFOC). She noted that KFOC is a patient-founded organization that funds nearly $4 millions of research per year. She also emphasized the role of KFOC in knowledge translation, noting that it takes a long time, 10-20 years, for research to go from discovery to bedside.
She then discussed KFOC’s strategic research framework that includes topics such as patient-oriented research (including patients in research), organ replacement strategies, prevention, nutrition, novel therapies and working across disciplines and pillars. She also noted that KFOC has launched an initiative called Horizons to help them create a strategic research framework.
Judith E. Fradkin
Director of the Division of Diabetes, Endocrinology, and Metabolic Diseases, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)

Dr. Judith E. Fradkin became the Director of Division of Diabetes, Endocrinology, and Metabolic Diseases (DEMD) at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) in 2000.
NIDDK research priorities include multi-centered clinical trials to evaluate new approaches to prevent and treat diabetes and its complications: scientific consortia to define the genetic and environmental triggers of diabetes, and molecular mechanisms underlying diabetes initiation and progression; and translational research to improve diabetes prevention and care and reduce disparities in diabetes treatment and outcomes.
In her presentation, Dr. Fradkin spoke about some of the mechanistic and therapeutic initiatives launched by the NIDDK, in order to offer opportunities for collaborations with Canadians researchers. These initiatives include: identification and characterization of rare/atypical diabetes phenotypes, for which the NIDDK has launched a nation-wide consortium. Also, translating genetic risk gene information into new molecules and pathways for therapeutic development. The NIDDK helped launch the Accelerating Medicines Partnership (AMP) T2D consortium to identify the effects of genetic changes that increase or decrease one's risk of developing type 2 diabetes and its complications. AMP T2D provides open access to query genetic and phenotypic data contributed by research consortia from many countries in North America, Europe, and Asia. Another initiative launched focuses on the comprehensive molecular profiling of human pancreas, adipose, kidney. The NIDDK also launched The Environmental Determinants of Diabetes in the Young (TEDDY) study to determine the environmental triggers of T1D. Another NIDDK initiative focuses on highly specific biomarkers (beta cell injury, diabetic foot ulcer healing), novel in vivo and in vitro platforms to model pathogenesis and therapy (organ on a chip, microphysiologic systems, novel mouse models) and beta cell replacement/regeneration. Lastly, she noted that huge resources have gone into artificial pancreas research, but the ultimate goal is replacement/regeneration. This area of research is a strength within Canada.
Jan Hux
President & CEO, Diabetes Canada

Jan Hux is the President and CEO of Diabetes Canada. Her background is as a clinician-scientist, trained as a general internist and health services researcher. Dr. Hux joined Diabetes Canada in 2012, and in her roles as Chief Science Officer and, more recently President, she has been a vocal advocate for the diabetes cause. She sees the need to create population impact through healthy public policy, evidence-informed health care and innovative research.
Dr. Hux began by speaking about the nature of the scientific research that is produced. She noted that usually the consumers of research are other scientists. However, in order to have a real impact on people suffering from disease, researchers should focus on patients being the ultimate consumers of their work. She discussed issues with knowledge translation and how to give primary care physicians the training to provide tools and new therapies discovered through research. She also discussed providing patients with access to the new research.
She then discussed Diabetes Canada’s shift in focus from direct service delivery towards population-level impact. She noted three key ways to achieve this: prevention using public policies and increasing awareness, better health outcomes via knowledge mobilization and working towards a cure by funding innovative research through open competitions. She noted that this shift will raise complicated questions, which will require data resources to answer. She believes that integrating data from various platforms will provide answers to much more complicated questions than any data bank can answer on its own.
Hugh O’Brodovich
Member, Board of Directors, Cystic Fibrosis Canada

Hugh O’Brodovich obtained his MD and training in pediatrics and pulmonology at University of Manitoba. Until his retirement, he had an active research program and paediatric pulmonary clinical practice. He is an elected Fellow of both the Canadian Academy of Health Sciences and American Association for the Advancement of Science. As a Stanford University Professor Emeritus, he lives in Canada and serves on the Scientific Advisory Board of AIT Therapeutics, Board of Directors of the Province of Ontario’s Central West Local Health Integration Network and Board of Directors of Cystic Fibrosis Canada (CFC). CFC has a focus on CF-related diabetes (CFRD).
Dr. O’Brodovich began his presentation by stating that CFC supports programs in clinical care, research, advocacy and a nation-wide registry. He emphasized that there is an established link between cystic fibrosis (CF) and diabetes. As reviewed by Bridges et al, approximately half of all adult CF patients have CFRD (Bridges et al 2018) and prior to lung transplantation, diabetes has been diagnosed in 63% of patients with cystic fibrosis (Belle-van Meerkerk et al 2012). In 1997 the USA’s Cystic Fibrosis Foundation reported a 6-fold increase in mortality in CF patients with CFRD (Cystic Fibrosis Foundation National Patient Registry. 1998. 1997 Annual Data Report. Bethesda, MD). In the Canadian CF patient registry, CFRD is similarly associated with increased mortality (Desai et al 2018). Even post-lung transplant CFRD is associated with increased mortality relative to CF patients without CFRD (Belle-van Meerkerk et al 2012). Women are most impacted by CFRD as the median age of survival for female CF patients is shortened from 47.0 years to 30.7 years if they had CFRD whereas median age for men was unaffected (Milla et al 2005). He also emphasized that the mechanism of CF related diabetes is still unclear. Quoting from Bridges et al (2018) “CFRD differs from Type 1 and Type 2 diabetes in several ways; there is a pattern of insulin deficiency with reduced and delayed insulin response to carbohydrates but a sparing of basal insulin that results in glucose abnormalities, which are frequently characterized by normal fasting glucose and postprandial hyperglycaemia. Insulin deficiency and hyperglycaemia, even at levels which do not reach the threshold for a diagnosis of diabetes, have an adverse impact on lung function and clinical status in people with cystic fibrosis”. Accordingly, CFC is interested funding research in this area.
References
Belle‐van Meerkerk, G., van de Graaf, E. A., Kwakkel‐van Erp, J. M., van Kessel, D. A., Lammers, J. J., Biesma, D. H. and de Valk, H. W. (2012). Diabetes before and after lung transplantation in patients with cystic fibrosis and other lung diseases. Diabetic Medicine, 29(8): e159-e162. doi: 10.1111/j.1464-5491.2012.03676.x
Bridges, N., Rowe, R., and Holt, R. I. G. (2018). Unique challenges of cystic fibrosis‐related diabetes. Diabetic Medicine, 35: 1181–1188. doi: 10.1111/dme.13652
Desai, S., Wong, H., Sykes, J., Stephenson, A. L., Singer, J., and Quon, B. (2018). Clinical Characteristics and Predictors of Reduced Survival for Adult-diagnosed Cystic Fibrosis. Analysis of the Canadian CF Registry. Annals of the American Thoracic Society, 15(10): 1177-1185. doi: 10.1513/AnnalsATS.201801-037OC
Milla, C. E., Billings, J., and Moran, A. (2005). Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care, 28(9): 2141-2144. doi: 10.2337/diacare.28.9.2141

Dr. Charu Kaushic moderating the discussion of the partner panel
Partner Panel Discussion
During the discussion, one participant commented on the success of the Canadian Partnership Against Cancer (CPAC), and asked whether there will there be something similar for diabetes. In response, it was noted that in the past many organizations have been focused on competing for donor dollars, attribution and credit for discovery. However, people have realized that working alone hinders progress and that by working together, we are able to accomplish much more. It was also stated that Diabetes Canada, CIHR and JDRF have worked together for a long time and will continue to do so. Although each partner has different strategies and goals, there is a lot of overlap, and working together on these goals is crucial.
In response to a question regarding collaboration between the NIH and CIHR, it was noted that many Canadian researchers have competed successfully for NIH support and that there are a number of ways in which CIHR and NIH collaborate. However, there are challenges in developing joint funding mechanisms to support collaborations between researchers. Also, there are issues with infrastructure coordination and there needs to be more open data and open access to data.
One presenter noted that in order to have effective partnerships, attribution is important and must be made explicit. Publications should acknowledge all funders because this allows health charities to demonstrate to donors where their money is being spent.
The partners were then asked what about the workshop intrigued them the most. Dr. Hux (Diabetes Canada) expressed optimism towards finding a cure, especially the potential of islet biology research. She was also intrigued by the discussion on open competitions and researchers learning to manage and become partners to overcome complex challenges. Similarly, Dr. Dunne (JDRF) was interested in the discussions on islet biology, Beta-cell survival and regeneration, immune regulation and prevention of type 1 diabetes. She also mentioned that Canada has a great infrastructure in place to build on these research areas. Ms. Fowler (KFOC) was intrigued by the discussions on complications, prevention and the impact of diet on diabetes. She also expressed interest in diabetes in indigenous populations, noting that indigenous health could be included as an overarching theme in this initiative and not be seen as a separate issue. Dr. Fradkin (NIDDK) noted that islet biology is a ripe opportunity for expansion: however, she also stated that there needs to be a greater focus on diabetes complications, other than cardiovascular disease, e.g., fatty liver disease. She also mentioned the success of TrialNet and wants to expand its reach. Lastly, Dr. O’Brodovich (CFC) mentioned that pigs and ferrets are increasing used as model organisms for CF and there is a move away from mice models. He also expressed interest in the prevention and modification of CFRD given its significant effect on morbidity and mortality in CF patients.
Small Group Discussions
Small Group Discussions And Dot-Mocracy: Part 1
Four small break-out groups were formed; each group to generate 5 scientific priorities that could be addressed through a strategic research initiative. Groups had flip chart paper, markers and instructions to report back. During the report back session, some of the topics identified were grouped together as they were deemed to be similar by the groups presenting. After the larger group had reconvened, each person was provided with 5 “sticky dots” to assign to the priorities that they deem most important. The results below provide a summary of this “dot-mocracy” exercise. This exercise was then repeated later in the day (Round 2), although some meeting participants had left early so the denominators are not equal. The results are presented in Table 1.
Table 1: Scientific priorities
| Priorities | Description | Round 1 | Round 2 |
|---|---|---|---|
| Prevention and modification of disease |
|
24 | 20 |
| Physiological insulin replacement |
|
20 | 17 |
| Islet Biology |
|
20 | 15 |
| Physiology |
|
19 | 16 |
| Genetics |
|
14 | 12 |
| Implementation Science |
|
14 | 11 |
| Complications of Diabetes |
|
10 | 4 |
| Hypoglycemia |
|
7 | 6 |
| Environment beyond genetics |
|
4 | 0 |

Results of part 1 of the dot-mocracy exercise
Small Group Discussions And Dot-Mocracy: Part 2
In the second small group discussion, participants were asked what they see as the top 3 priorities for potential structures needed to enhance collaboration and advance research in the field. Again, participants were given time to discuss this within their respective small groups, and then they were asked to report back and indicate what they deemed as the most important/highest priority among those options identified to enhance collaboration. The results below provide a summary of this “dot-mocracy” exercise.
Table 2: Priorities to enhance collaboration
| Priorities | Description | Green dots |
|---|---|---|
| Distributed national cores |
|
19 |
| Registry |
|
12 |
| Mechanisms to bring people together |
|
9 |
| Training and Resources |
|
9 |
| Collaboration partnerships with private sector |
|
6 |
| Implementation |
|
5 |
| Institutional support |
|
3 |
| Clinical trial units |
|
2 |
| Collaboration |
|
1 |

Researchers performing part 2 of the dot-mocracy exercise
Conclusion and Closing Remarks
Norman Rosenblum provided a brief overview of next steps in the development of this strategic research initiative. He noted that the 100th anniversary of the discovery of insulin will occur in 2021, and this Workshop is the first step in seeking input from the research community. There is a need for further discussion of this initiative with the INMD Institute Advisory Board, and potential partners and collaborators, both within CIHR and externally. These discussions will help to shape the initiative and hopefully provide more resources to fund the research.
Dr. Rosenblum concluded the meeting by thanking all the Workshop participants, and in particular, he thanked the Disruptors who were effective in encouraging participants to think creatively about how we can accelerate research progress in diabetes. He also provided a special thanks to Diabetes Canada, for providing the opportunity for CIHR-INMD to host the Workshop in conjunction with their annual meeting, and thanked representatives from other partner organizations and health charities who provided input at the Workshop. Finally, he thanked INMD staff who worked hard to plan the Workshop.
Contact Us
For more information about the 100 Years of Insulin Diabetes Workshop, please visit our website or contact us at: inmd.comms@sickkids.ca.
Appendix 1: Agenda
CIHR - INMD INVITATIONAL Workshop:
100 Years of Insulin: What’s Next?
Wednesday, October 10, 2018 8:00 am to 5:00 pm
Halifax Convention Centre
The purpose of this workshop is to seek input from the Canadian diabetes research community on the foci, structure and partnership opportunities to inform a strategic research initiative to commemorate the 100th anniversary of the discovery of insulin.
Objectives
The primary objectives of this Workshop are to bring together researchers and other stakeholders to:
- Identify the Canadian strengths from within and external to the diabetes research community that could be leveraged to support a strategic research initiative on the mechanistic and therapeutic aspects of diabetes research.
- Define the scientific priorities of the Canadian diabetes strategic research initiative.
- Identify the potential structures needed to enhance collaboration and advance research in the field.
- Identify a list of potential partners, including international collaborators, who could work with CIHR to support this research initiative.
| 8:00 AM - 9:00 AM | Breakfast | |
|---|---|---|
| 9:00 AM - 9:15 AM | Opening Welcome, Agenda Overview and Introductions | Norm Rosenblum |
| 9:15 AM - 9:45 AM | Setting the Scene – Reflecting on Canadian Diabetes Research Strengths and Opportunities | Dan Drucker |
| 9:45 AM - 10:45 AM | The Disruptors: Leveraging Innovation from Inside and Outside the Field of Diabetes Research | Anna Gloyn Robert Ratner Matthias von Herrath |
| 10:45 AM - 11:00AM | Nutrition Break | |
| 11:00 AM - 12:00 PM | Moderated Panel Discussion: Dan Drucker Anna Gloyn Robert Ratner Matthias von Herrath |
Moderator: Norm Rosenblum |
| 12:00 PM - 1:00 PM | Lunch | |
| 1:00 PM - 1:15 PM | Recap of Key Points from the Disruptors: Promising Scientific Opportunities |
Norm Rosenblum |
| 1:15 PM - 1:45 PM | Small Group Work to Define the Scientific Priorities of the Initiative | - |
| 1:45 PM - 2:15 PM | Report Back and Dot-mocracy | Moderator: Chris McMaster |
| 2:45 PM - 3:45 PM | Partner Perspectives: Diabetes Canada – Jan HuxJDRF – Jessica Dunne KFOC – Elisabeth Fowler NIDDK – Judy Fradkin Cystic Fibrosis Canada – Hugh O'Brodovich |
Moderator: Charu Kaushic |
| 3:45 PM - 4:00 PM | Health Break | |
| 4:00 PM - 4:30 PM | Small Group Work
|
- |
| 4:30 PM - 4:45 PM | Report Back and Dot-mocracy | Moderator: Chris McMaster |
| 4:45 PM - 5:00 PM | Closing Remarks – Next Steps-Thank you | Norm Rosenblum |
Appendix 2: Meeting Participants
| Name | Affiliation |
|---|---|
| Gillian Booth | St. Michael’s Hospital, University of Toronto |
| Jean-Pierre Després | Québec Heart and Lung Institute, Université Laval |
| May Faraj | Institut de recherches cliniques de Montréal, Université de Montréal |
| James Johnson | University of British Columbia |
| Christopher Kennedy | University of Ottawa |
| Timothy J. Kieffer | University of British Columbia |
| Gregory Korbutt | University of Alberta |
| Tony Lam | Toronto General Research Institute, University of Toronto |
| Gary Lewis | University of Toronto |
| Francis Lynn | BC Children’s Hospital Research Institute, University of British Columbia |
| Patrick MacDonald | Alberta Diabetes Institute, University of Alberta |
| André Marette | Université Laval |
| Cristina Nostro | Toronto General Hospital Research Institute, University of Toronto |
| Vincent Poitout | Université de Montréal |
| Rémi Rabasa-Lhoret | Institut de recherches cliniques de Montréal, Centre Hospitalier de l’Université de Montréal, Université de Montréal |
| Peter Senior | University of Alberta |
| Robert Sladek | McGill University |
| Bruce Verchere | BC Children’s Hospital, University of British Columbia |
| Minna Woo | University Health Network, University of Toronto |
| Partners | |
|---|---|
| Jessica Dunne | JDRF |
| Dave Prowten | JDRF Canada |
| Elisabeth Fowler | Kidney Foundation of Canada |
| Judith E. Fradkin | National Institute of Diabetes and Digestive and Kidney Diseases |
| Jan Hux | Diabetes Canada |
| Hugh O’Brodovich | Cystic Fibrosis Canada |
Appendix 3: CIHR Scientific Directors And Staff
| Scientific Directors | |
|---|---|
| Norman Rosenblum | CIHR Institute of Nutrition, Metabolism and Diabetes |
| Charu Kaushic | CIHR Institute of Infection and Immunity |
| Christopher McMaster | CIHR Institute of Genetics |
| CIHR Staff | |
| Étienne Richer | Associate Director Institute of Genetics |
| Gerrilynn Manitowabi | Project Officer Institute of Indigenous Peoples' Health |
| Elisabeth Page | Assistant Director Institute of Infection and Immunity |
| Tanya Gallant | Assistant Director Institute of Musculoskeletal Health and Arthritis |
| Mary-Jo Makarchuk | Assistant Director Institute of Nutrition, Metabolism and Diabetes |
| Keeley Rose | Project Manager Institute of Nutrition, Metabolism and Diabetes |
| Hasnain Saherawala | Project Analyst Institute of Nutrition, Metabolism and Diabetes |
| Christine Dhara | Business Officer and Event Planner Institute of Nutrition, Metabolism and Diabetes |
| Kelly Taylor | Director General Program Design and Delivery |
| Dale Dempsey | Manager Program Design and Delivery |
| Kim Banks Hart | Manager Strategic Partnerships and International Relations |
- Date modified: